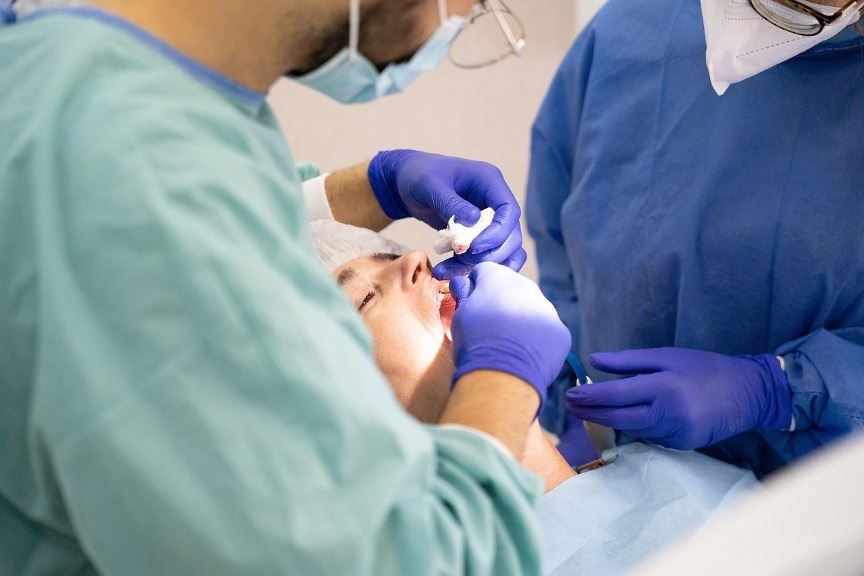
The Anterior Growth Guidance Appliance is a dental device that has been the subject of numerous lawsuits due to alleged harm caused to patients. Marketed as a retainer-like device, some dentists promote it to expand adult patients’ jawbones, enhance facial appearance, and treat conditions like sleep apnea. However, patients involved in the lawsuits claim to have suffered significant damage, including damaged gums, bone erosion, and tooth loss, as a result of using the AGGA.
If an AGGA device has harmed you and you want to file a lawsuit, or if you’re considering using an AGGA, keep reading to learn about the controversy surrounding this dental device.
The Negative Effects of the Anterior Growth Guidance Appliance
The Anterior Growth Guidance Application has gained popularity recently as an alternative approach to orthodontic treatments, particularly for individuals seeking maxillary growth or correction of certain bite issues. The AGGA device is intended to promote forward growth and development of the jaw, which can reportedly improve facial features and treat conditions like sleep apnea. While no precise data is available on the exact number of individuals with AGGA dental devices, it is estimated that at least 10,000 patients across the United States have received this treatment.
However, AGGA dental devices have been controversial due to the negative side effects reported by some users. The primary allegation is that the use of an AGGA can lead to various adverse effects and injuries, such as jaw pain, temporomandibular joint (TMJ) issues, bite misalignment, tooth movement, facial disfigurement, and nerve damage. Some users have reported that the device caused or exacerbated jaw-related problems, leading to pain and discomfort. Additionally, the claims of bite problems and tooth movement suggest potential issues with the alignment and stability of the teeth.
Are AGGA Dental Devices Safe for Use?
The safety of the AGGA dental device is a matter of debate and subject to individual experiences. While some users report positive outcomes and satisfactory results from using the AGGA device, others have experienced adverse effects. The safety of the AGGA device depends on various factors, including proper diagnosis, treatment planning, and professional oversight during its use.
It is important to note that the AGGA device is a specialized treatment modality used in dentistry and orthodontics to address specific jaw growth and alignment conditions. Like any medical or dental treatment, there can be risks and potential side effects associated with its use. However, the overall safety and effectiveness of the AGGA device may depend on factors such as the skill and experience of the healthcare professional prescribing and monitoring its use, as well as the individual patient’s specific circumstances and response to the treatment.
Are Anterior Growth Guidance Appliances FDA Approved?
The FDA’s regulatory oversight typically extends to drugs, medical devices, and food products, while dental appliances like AGGAs fall under the purview of dental boards and regulatory bodies. Therefore, the regulation of the AGGA lies within the jurisdiction of dental professionals and the dental industry itself.
While the FDA does not provide specific approval for AGGAs, dental professionals and manufacturers are expected to adhere to certain guidelines and standards set by professional organizations and regulatory bodies to ensure the safety and efficacy of these devices.
Lawsuits Surrounding AGGA Devices
Lawsuits surrounding AGGA devices were filed as early as 2000. However, in the past three years, at least 20 patients have filed lawsuits against the manufacturers and designers of AGGA dental devices. Suppose you have experienced negative health complications from the use of an AGGA device. In that case, you may also be able to file a product liability lawsuit to receive compensation for your injuries.
AGGA device lawsuits typically involve injury claims, product liability, and professional negligence. Individuals who have experienced adverse effects or unsatisfactory outcomes from AGGA devices may seek legal recourse to seek compensation for their damages. This legal action seeks to hold the company accountable for any injuries or damages caused by its product.
AGGA Product Liability Lawsuit
In a product liability lawsuit, individuals allege that the AGGA device was defective or inherently dangerous, leading to their injuries. These lawsuits may assert claims against the manufacturers, distributors, or sellers of the AGGA device, seeking compensation for medical expenses, pain and suffering, and other related damages.
AGGA Professional Negligence Lawsuit
Another type of lawsuit may arise from professional negligence or malpractice claims against dentists or orthodontists who prescribed or administered the AGGA device. In such cases, plaintiffs may allege that the healthcare professionals failed to adequately inform them about the treatment’s potential risks and side effects or that they deviated from accepted standards of care in fitting or monitoring the AGGA device. These lawsuits aim to hold healthcare providers accountable for any harm or injuries caused by the AGGA device and seek compensation for the resulting damages.
Elements of an AGGA Liability Lawsuit
Injured patients must establish certain elements to successfully sue for damages associated with the use of an AGGA dental device. Firstly, they must demonstrate that they used the AGGA dental device as intended or as directed by the company or healthcare professional. Secondly, they need to show that the device was defective or unreasonably dangerous due to a design flaw, manufacturing defect, or inadequate warnings or instructions. Finally, they must establish a direct link between the use of the device and their injuries, proving that the device caused harm.
By filing a lawsuit against the company, injured patients may seek compensation for various damages, including medical expenses, pain and suffering, lost wages, and any long-term or permanent disabilities resulting from the AGGA dental device. The compensation awarded will depend on factors such as the severity of the injuries, the impact on the patient’s life, and the evidence presented during the legal proceedings. It is crucial for affected patients to consult with an experienced personal injury attorney specializing in product liability cases to navigate the legal complexities and maximize their chances of a successful lawsuit.
Current Status of the AGGA Criminal Investigation
Federal prosecutors have initiated a criminal investigation to determine the safety and potential harm caused by the AGGA device, as multiple lawsuits have alleged severe damage to patients, including damaged gums, eroded bone, and tooth loss. The U.S. Attorney’s Office for the Western District of Tennessee and the U.S. Department of Justice oversee the criminal investigation.
In response to the criminal investigation, a motion was filed in federal court to delay the largest lawsuit until the outcome of the criminal proceedings. The motion was filed by attorneys representing AGGA inventor Dr. Steve Galella, his company, the Facial Beauty Institute, and AGGA manufacturer Johns Dental Laboratories. The court filing indicates that the investigation is being conducted with the intention of potentially bringing criminal charges against the defendants, although the specific charges have not been specified. The attorneys for both the plaintiffs and the defendants have chosen not to comment on the ongoing investigation.
Though not specifically covered under the FDA, the safety of the AGGA device is also being investigated by the FDA. The AGGA’s lack of registration with the FDA has raised questions about its compliance with regulatory standards.
How to File Your AGGA Lawsuit
If you are considering filing an AGGA lawsuit, consulting with a qualified attorney specializing in medical device litigation is important. Start by researching law firms or attorneys with experience handling product liability or medical malpractice cases. Look for firms with a successful track record in handling similar lawsuits and representing plaintiffs who have suffered harm from dental devices like the AGGA.
Once you have identified potential attorneys or law firms, schedule a free consultation to discuss your case. During the consultation, provide all relevant information and documents related to your AGGA treatment and the resulting harm or injuries you have experienced. The attorney will evaluate the strength of your case, assess the potential legal claims, and explain the legal process involved in filing a lawsuit against the responsible parties.
Morris & Dewett does not typically represent mass tort cases. If you are a Louisiana or Texas resident, we may be able to provide a recommendation.